Renalys Pharma’s Sparsentan Secures the MHLW’s Orphan Drug Designation to Treat Primary IgA Nephropathy
Shots:
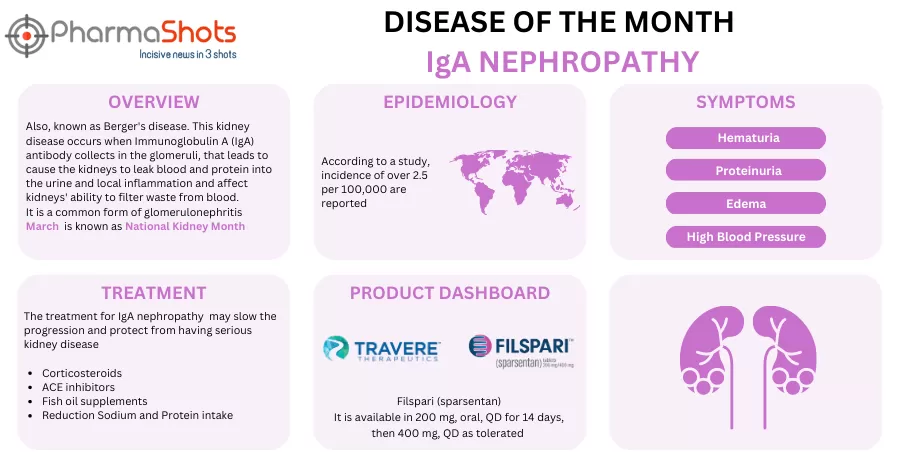
- The Japanese MHLW has granted ODD to sparsentan, being investigated under P-III study in Japan for treating IgA nephropathy. It was FDA approved under brand name Filspari for slowing kidney function among adults in Sep 2024
- Renalys secured its development & commercialization rights from Travere Therapeutics across Japan, South Korea, Taiwan, Brunei, Cambodia, Indonesia, Laos, Malaysia, Myanmar, the Philippines, Singapore, Thailand & Vietnam as per an agreement signed in Jan 2024
- Filspari (QD, oral) is a non-immunosuppressive medication for IgAN that blocks endothelin-1 and angiotensin II pathways to reduce glomerular injury
Ref: Renalys | Image: Renalys
Related News:- Travere Therapeutics’ Filspari (sparsentan) Receives the US FDA’s Full Approval for Treating IgA Nephropathy
PharmaShots! Your go-to media platform for customized news ranging for multiple indications. For more information connect with us at connect@pharmashots.com
Click here to read the full press release

Disha was a content writer at PharmaShots. She is passionate and curious about recent updates and developments in MedTech and Pharma industry. She covers news related to clinical trial results and updates. She can be contacted at connect@pharmashots.com.